OUR PRODUCTS
CMB Healthcare Devices
Portable CMB Devices
R&D
M20 has demonstrated verified technological capabilities and
outstanding product development skills through continuous research and development.
Empty Date

2017
Development of Muscle Strengthening Program and CMB Technology Internalization
Empty Date

2018
Launched Integrated Smart Healthcare Platform 'MYO'
Empty Date

2019
Participated in FIBO 2019, World's Largest Fitness Exhibition
Empty Date

2020
Participated in CES 2020
Empty Date

2020
Selected for 2020 Good Design Award by the Ministry of Trade, Industry and Energy
Empty Date

2021
Obtained Certification as a Technology Innovation-Driven Small and Medium Enterprise (Inno-biz) and Medical Device GMP Certification
Empty Date

2022
Finalist at IDEA 2022, One of the World's Top 3 Design Awards
Empty Date

2023
MYO Home, First Domestic EMS Manufacturer to Obtain US FDA Medical Device Approval
Empty Date
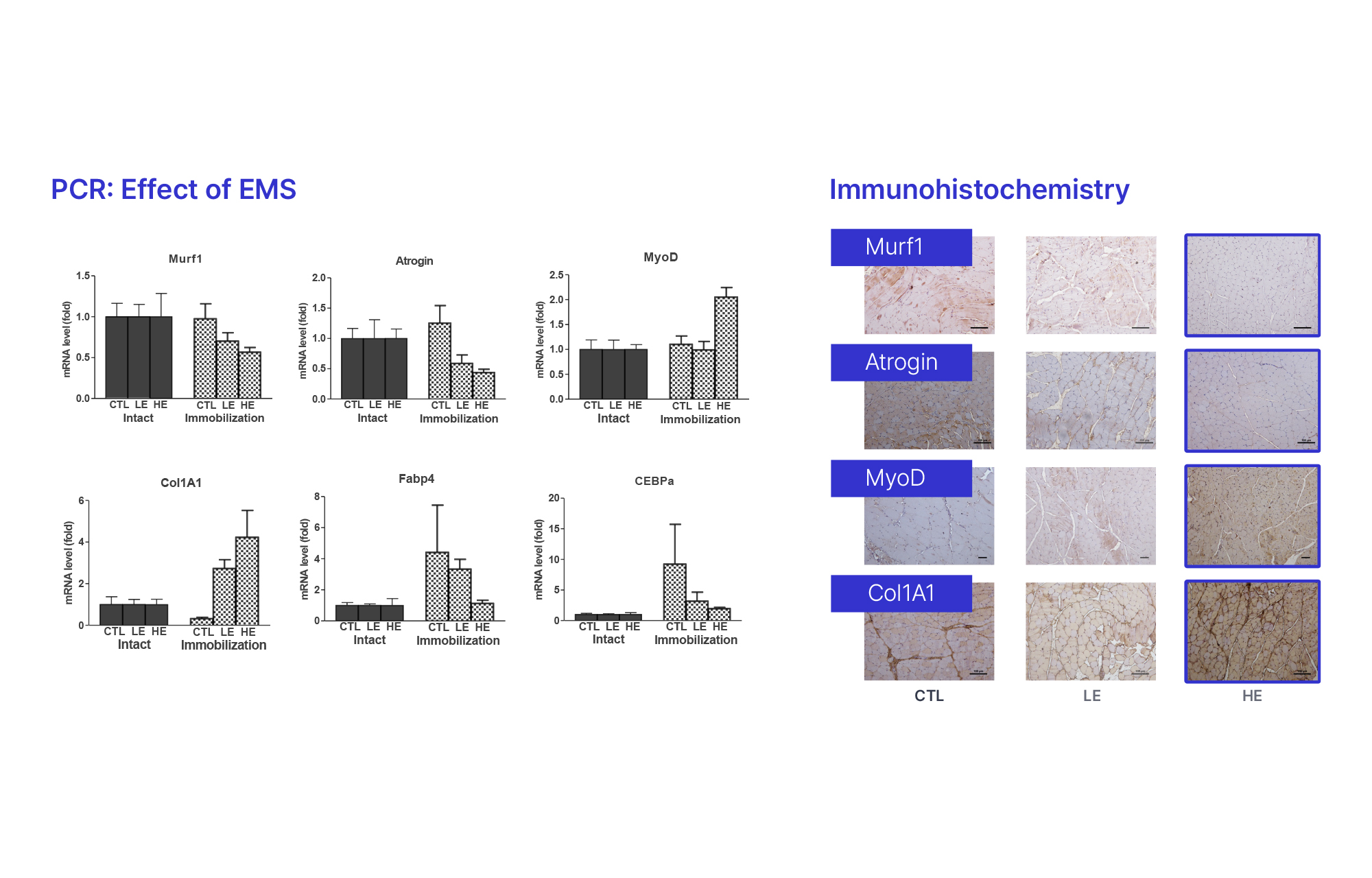
2024
Pre-clinical Animal Studies Confirmed Low to Medium Frequency Electrical Stimulation Significantly Effective for Muscles
Empty Date
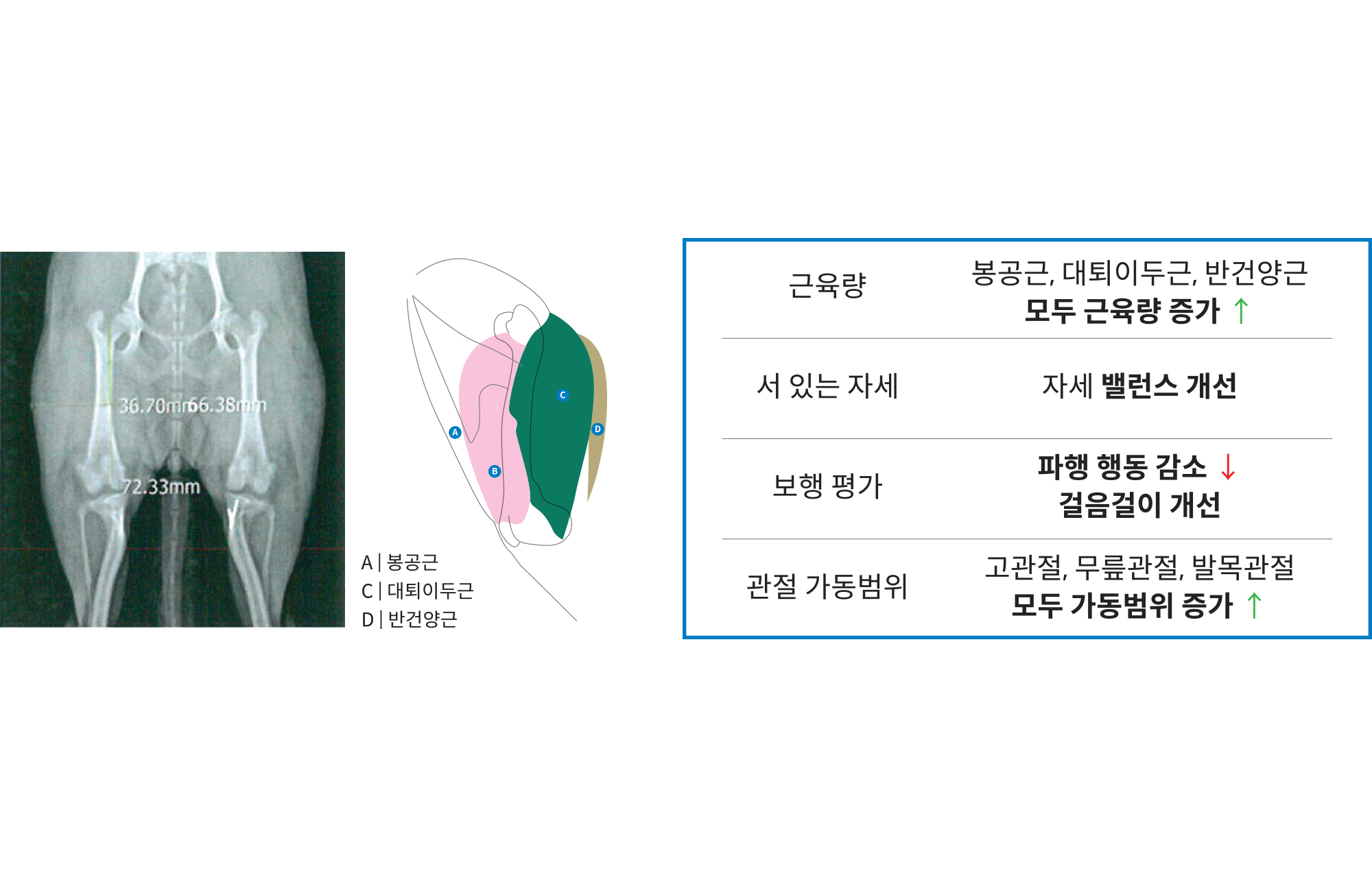
2024
Clinical Experiments with VIP Animal Medical Center Proved Improvement in Companion Dogs' Conditions
Empty Date
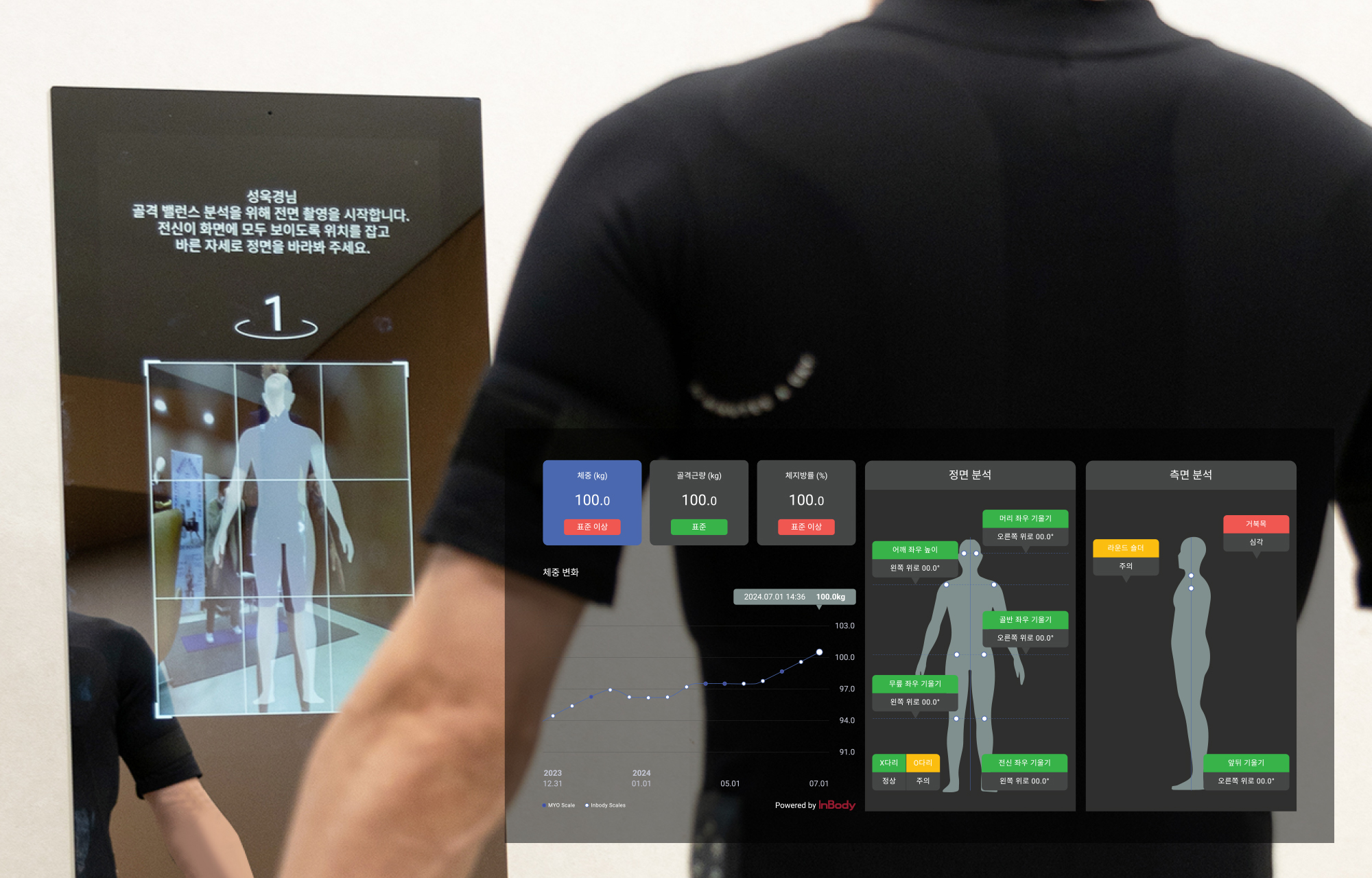
2024
Advanced Body Composition Analysis/Skeletal Balance Analysis Function
(Collaboration with INBODY/REEMO)
(Collaboration with INBODY/REEMO)
PATENT & CERTIFICATION
All core technologies of M20 are independently researched, developed, and commercialized, and we will secure
R&D capabilities through continuous investment.

Acquired FDA Certification

Growth plate stimulation management system using biosignals and deep learning algorithms

Sportswear that can provide electrical stimulation

Non-face-to-face health video training platform

Growth plate stimulation device

Marketing method using smart mirrors and golf coaching system

CE Certified

KC Certified

EMS Treatment device method for companion animal

System for multi controlling generator of electrical muscle stimulation employing filed programmable gate array

System for equalization of muscle stimulation between virtual influencer and user employing AI Motion desetion and VR/AR







